There are several reasons we take thoracic radiographs. These include a noted heart murmur, screening for metastatic disease, evaluating for clinical problems such as a fever of unknown origin or most commonly, for the evaluation of a patient coughing or having respiratory distress. In this article, we will review lung patterns and distributions of common pulmonary diseases while putting the clinical picture together.
Radiographic Lung Patterns in Dogs and Cats
Lung patterns are simply the radiographic appearance of diseases in the lungs. See Table 1 for differential diagnosis for common lung patterns in dogs and cats.
Common lung patterns include:
- A bronchial pattern is diffuse thickening of the airway walls giving the appearance of thick lines and rings throughout the lungs. A bronchial pattern is important to recognize, because, while it may be a normal variant in an aged patient, it may also be due to a specific subset of diseases. These include bronchitis, asthma, Kennel cough, Mycoplasma pneumonia, and parasitic pneumonitis, such as heartworm or lung worm disease. Rarely there can be mineralization of the airways with certain metabolic disease (e.g. hyperadrenocorticism).
- An unstructured interstitial pattern is simply increased soft tissue opacity in the lungs that partially obscures blood vessel margins, due to the presence of edema, pus, blood, or other material in the lungs (Figure 1). The appearance of an interstitial pattern itself is not specific for certain diseases. Instead, the distribution of the interstitial pattern is suggestive of certain differential diagnoses. There are numerous diseases in the lungs that can manifest as an interstitial pattern. These include pulmonary edema, pneumonia, a pulmonary thromboembolism, hemorrhage, or fibrosis.
- An alveolar pattern is more severe than an interstitial pattern, in that it is an area of increased soft tissue opacity in the lungs that completely obscures pulmonary blood vessels (Figures 2 and 3). Air filled bronchi may be evident as “air bronchograms” unless the airways are also filled with infiltrate or are collapsed. If the alveolar infiltrate abuts the lung margins, then there will be a lobar sign, which is the appearance of a soft tissue opaque lung abutting normal air-filled lung. Differential diagnoses for an alveolar pattern are mostly the same as those for an interstitial pattern.
- A structured interstitial pulmonary pattern is otherwise known as nodules. Nodules are round or ovoid soft tissue or mineral opaque structures in the lungs. Differential diagnoses for nodules seen in the lungs include neoplasia, fungal disease, parasitic cysts or granulomas, fluid/blood filled bullae, pulmonary osteomas, or end-on blood vessels.
- A vascular pattern is when enlarged and/or tortuous blood vessels result in increased soft tissue opacity in the lungs. Differential diagnoses for enlargement of the pulmonary vasculature include heartworm disease, left sided congestive heart failure, or left to right sided cardiac shunting, such as a patent ductus
- Other less common pulmonary findings include bullae or air-filled ovoid structures or a vesicular pattern, which is usually only associated with lung lobe torsion. For the purpose of this article, we will focus on interstitial and alveolar patterns in our coughing and distressed patients, and touch on bronchial patterns.
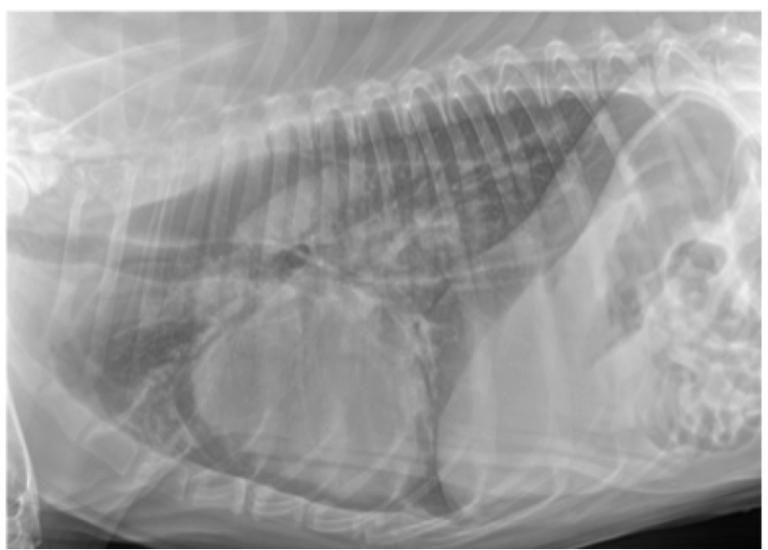
Figure 1. Lateral thoracic radiograph from a dog showing an unstructured interstitial pattern. Note the increased soft tissue opacity in the lungs that partially obscures blood vessel margins.
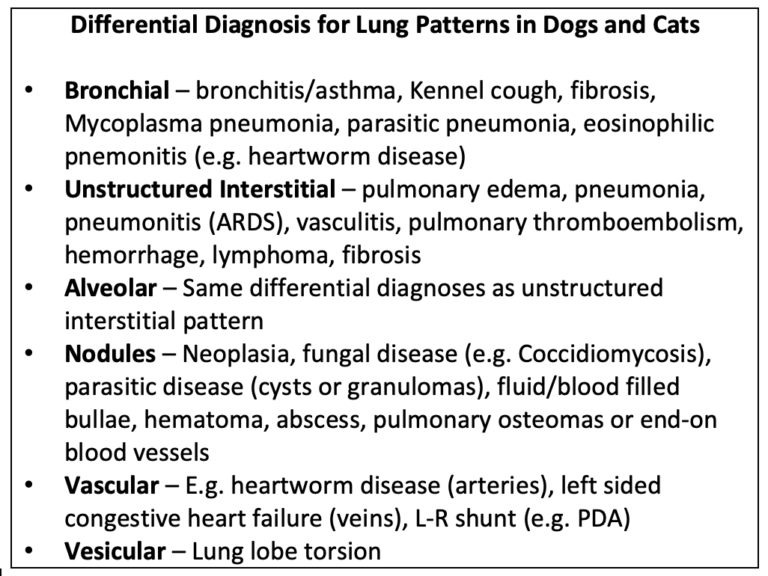
Table 1. Differential diagnosis for common lung patterns in dogs and cats.
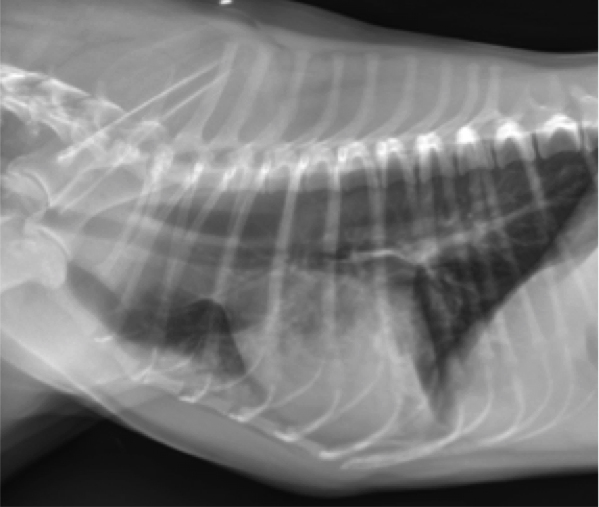
Figure 2. Lateral thoracic radiograph from a dog with a ventral alveolar pattern. Note the lobar sign with the caudal lung lobe.
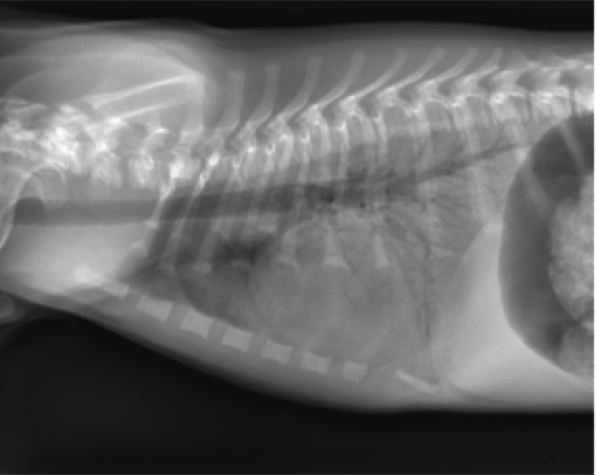
Figure 3. Lateral thoracic radiograph from a dog with a caudodorsal alveolar pattern. Note the air bronchograms throughout the abnormal lung.
Radiographic Distribution of Pulmonary Patterns in Dogs and Cats
The distribution of pulmonary disease is of utmost importance when determining the most likely differential diagnoses. Important examples of radiographic lung patterns include the following:
Cranioventral Interstitial or Alveolar Pattern
When we have a patient with a cranioventral interstitial or alveolar pattern, differential diagnoses include aspiration pneumonia, atelectasis, or hemorrhage or pulmonary thromboembolism. Less common causes for this appearance are masses, abscesses, or lung lobe torsion.
Pneumonia is a diagnosis that will commonly be made in dogs with a cough or hacking, with or without evidence of a fever. It is important to look for pneumonia in patients that may have been vomiting, have undergone anesthesia, prolonged recumbency, have megaesophagus, immune compromise or airway susceptibility (e.g. prior laryngeal paralysis tie-back surgery). Pneumonia will appear radiographically as an interstitial to alveolar pattern in the cranioventral aspects of the lungs. It may be in a focal lung lobe (most commonly the right middle) or in the ventral aspect of all of the lungs. It is important to include left and right lateral radiographs, as it may only be detectable on one side, depending on which lung is diseased. Because of the ventral distribution, it may not be evident on the ventrodorsal projections, because of the overlying mediastinum.
If there is focal or multifocal alveolar infiltrate, it will commonly contain air bronchograms. It is important to evaluate the area overlying the cardiac silhouette on the lateral projection, because this is a common location for focal aspiration pneumonia. Sometimes, with more severe cases, there may be concurrent, mild pleural effusion. If we diagnose a patient with aspiration pneumonia, based on radiographic appearance, fever, neutrophilia and/or a history supportive of this disease, then the patient should be treated with antibiotics. Ideally, antibiotics should be chosen based on culture of the airways, but typically, empiric therapy will be chosen without a culture in non-complicated and non-recurrent cases.
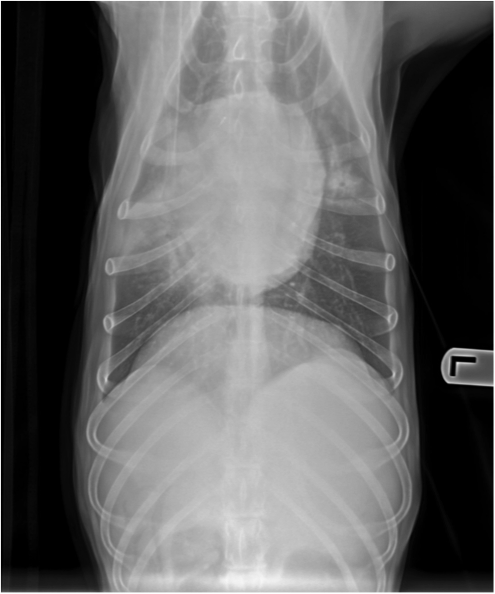
Figure 4. Ventodorsal thoracic radiograph from a dog with aspiration pneumonia. Note the consolidation of the right middle lung lobe.
Common organisms isolated from dogs and cats with lower respiratory disease include E.coli, Pasteurella spp., Streptococcus spp,B.bronchiseptica, Enterococcus spp., Mycoplasma spp., S. pseudintermedius and other coagulase‐positive Staphylococcus spp., and Pseudomonas spp. Although there has not been enough research to determine the duration of antibiotics, typically they are administered for 4 to 6 weeks. It is recommended to recheck three-view thoracic radiographs in 10 to 14 days after initiating antibiotics. Antibiotics should be continued for 1-2 weeks after radiographs are normal.
If the patient has had recent trauma or is coagulopathic (e.g. with rodenticide toxicity or DIC), then hemorrhage is a more likely differential diagnosis. Hemorrhage commonly settles into the dependent lungs and is therefore ventral. However, with trauma, pulmonary contusions are usually in the area of impact.
Pulmonary contusions are commonly noted adjacent to rib or vertebral fractures or adjacent to puncture wounds. Radiographic evidence of pulmonary contusions may appear or worsen after 24 hours of trauma and resolve within a few days in recovering patients. Discrete soft tissue opacities that remain when the overall lung pattern improves are more likely to be hematomas and take longer to resolve. With trauma, there may also be hemothorax and/or pneumothorax. This is important to identify, as thoracocentesis may be indicated. In a retrospective analysis of 143 dogs with pulmonary contusions secondary to motor vehicle accidents, dogs with more severe thoracic radiographic changes on presentation were more likely to need to receive oxygen, received oxygen for longer periods of time, and had a longer hospitalization time (Powell et al. 1999).
If there is no history or evidence of trauma, and no indication of pneumonia, then certain tests should be run to screen for causes of pulmonary hemorrhage. These include coagulation times, and a platelet count.
Another less likely cause for a ventral alveolar pattern is mucous plugging and atelectasis in conjunction with feline asthma. This will be in conjunction with a diffuse bronchial pattern due to bronchial thickening. Often times there will be air trapping due to asthma. This can cause the thorax to have an expanded “barrel shape” and may result in flattening of the diaphragm on the lateral images, and multiple cranially directed points to the diaphragm at the areas of muscular attachments, otherwise known as “tenting of the diaphragm.”
If radiographs and clinical signs are consistent with feline asthma, the patient should be treated with anti-inflammatory doses of glucocorticoids. Oxygen supplementation may be indicated in cases of acute distress due to feline asthma. Bronchodilators may be of benefit as well, but should not be used if there is concurrent cardiac disease. If clinical signs are persistent with treatment, airway sampling for cytologic evaluation, culture, Mycoplasma testing, as well as heart worm and lungworm testing should be considered.
Another, less common ventrally distributed pulmonary disease is lung lobe torsion. It may be an alveolar pattern, but a vesicular pattern, or vesicular emphysema, characterized by locally extensive small gas bubbles in the effected lung. The right middle lung lobe is the most commonly affected. There is usually concurrent pleural effusion. The torsion of the bronchus may be visible radiographically as a displaced or blunted airway. Otherwise, computed tomography is indicated for a definitive diagnosis. Once a diagnosis of lung lobe torsion is made, emergency surgery is indicated for lobectomy of the torsed lung.
Caudodorsal Distribution of an Interstitial or Alveolar Pattern
There is a different subset of diseases that cause caudodorsal distribution of an interstitial or alveolar pattern. These include cardiogenic pulmonary edema, non-cardiogenic pulmonary edema, hematogenous, viral or atypical pneumonia, pulmonary contusions, pulmonary thromboembolic disease, and neoplasia.
Cardiogenic pulmonary edema presents as interstitial to alveolar infiltrate in the peri-hilar and caudodorsal aspects of the lungs. The pulmonary blood vessels in this area may be partially to completely obscured. Most of the time there will be concurrent pulmonary venous congestion, unless the patient is already receiving Lasix therapy. It is important to remember that patients with cardiogenic pulmonary edema are in left-sided congestive heart failure. Therefore, there should be evidence of cardiomegaly. This may be left sided cardiomegaly due to mitral valve disease, dilated cardiomyopathy or the appearance of a valentine or generally enlarged heart with cats with cardiomyopathy. The exception is in patients with a ruptured chordae tendinae. This is rare and will present with patients with severe congestive heart failure and a severe heart murmur. Other clinical signs of congestive heart failure should be present. These signs include a heart murmur, tachycardia, decreased perfusion (i.e. poor pulse quality and possible hypothermia).
The main radiographic finding in support of cardiac disease is cardiomegaly. It is important to be familiar with the normal cardiac silhouette in order to determine if there is specific chamber enlargement or generalized cardiomegaly. When using the vertebral heart score, a VHS > 10.7 or <9.2 is likely abnormal. This scale has to be used with caution, as some breeds of dogs (e.g. English bulldog) can have vertebral heart scores around 12 with no evidence of cardiac disease. This is why it is helpful to use subjective assessment for chamber enlargement or generalized cardiomegaly as well.
When using the clock-face analogy on ventrodorsal projections (Figure 5), the center of the clock is at the carina. The 11:30-12:30 o’clock position is the aortic arch. The 1-2 o’clock position is the main pulmonary artery. The 2-3 o’clock position is the left auricle. The 4-5 o’clock position is the left ventricle. The 6 o’clock position between the caudal principle bronchi is the left atrium. The 7-8 o’clock position is the right ventricle and the 9:30-11:30 o’clock position is the right atrium.
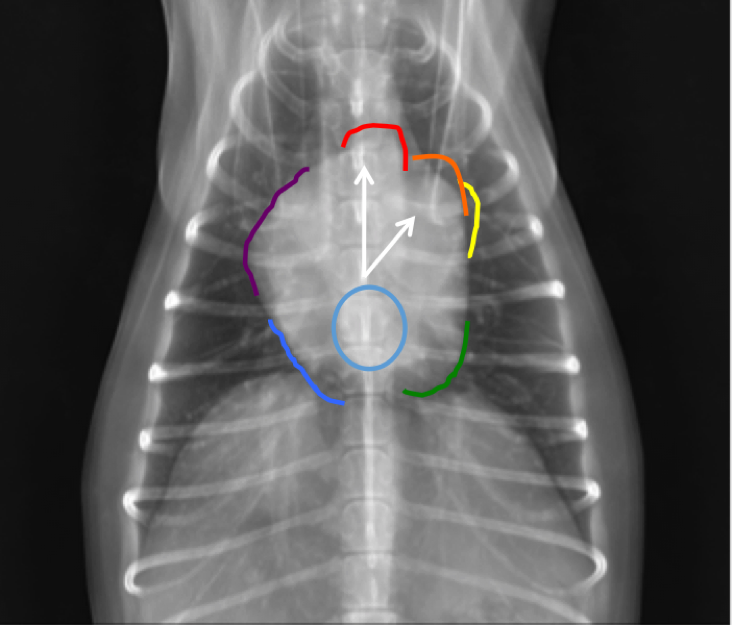
Figure 5. Vendrodorsal thoracic radiograph from a dog demonstrating the clock-face analogy. Note that 12 o’clock: Aortic Arch (red), 1 o’clock: Main Pulmonary Artery (orange), 2 o’clock: Left Auricle (yellow), 4-5 o’clock: Left Ventricle (green), 6 o’clock: Left Atrium (light blue) , 7-8 o’clock: Right Ventricle (Medium blue), 9-10 o’clock: Right Atrium (purple).
On the lateral projection (Figure 6), one can assess for left or right-sided cardiomegaly as well.
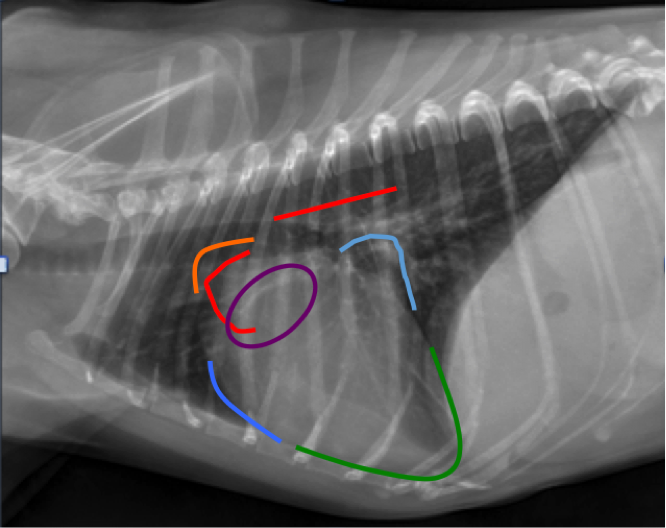
Figure 6. Lateral thoracic radiograph from a dog demonstrating the clock-face analogy. Note Aortic Arch (red), Main Pulmonary Artery (orange), Left Ventricle (green), Left Atrium (light blue), Right Ventricle (Medium blue), Right Atrium (purple).
If you drop an imaginary line (or draw a line) from the carina to the apex of the heart, 2/3 of the heart should be cranial to the line and 1/3 should be caudal in a normal patient (Figure 7). If there is more than 2/3 of the heart cranial to the line, then this is evidence of right-sided cardiomegaly. If there is greater than 1/3 caudal, then there is likely left sided cardiomegaly, specifically left atrial enlargement. These normals don’t always apply to cats, as their hearts sit more upright in the thorax. A normal feline heart should be ovoid or almond shaped. Rounding, particularly in the area of the atria, can indicate cardiomegaly.
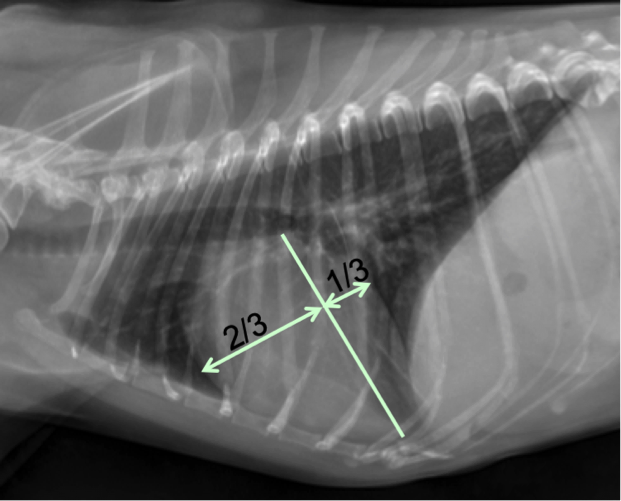
Figure 7. Lateral thoracic radiograph from a dog. If you drop an imaginary line (or draw a line) from the carina to the apex of the heart, 2/3rds of the heart should be cranial to the line and 1/3rd should be caudal in a normal patient (dogs).
Enlargement of the left atrium is the most common type of cardiomegaly identified radiographically. It is usually due to dilation of the left atrium associated with mitral valve disease. On the lateral projection, left atrial enlargement causes a soft tissue bulge or flattening of the caudodorsal aspect of the cardiac silhouette. Due to increased height of the left atrium, it can also cause dorsal displacement of the carina and principle bronchi (Figure 8). There can be selective displacement, and in some cases, compression of the left principle bronchus. These cases with bronchial compression secondary to left atrial enlargement often present with a cough.
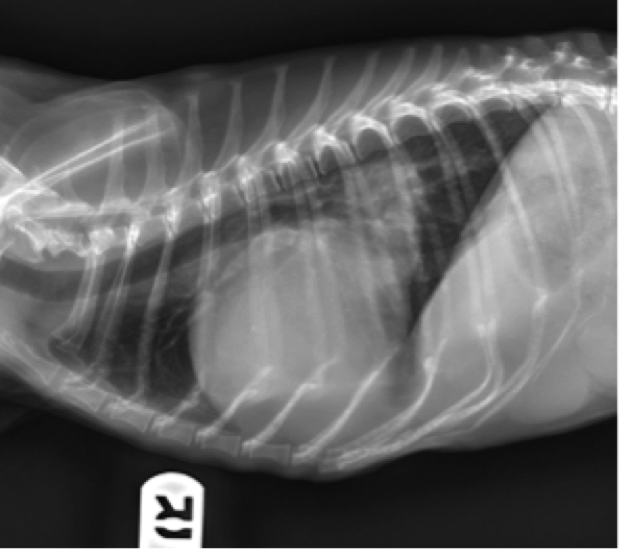
Figure 8. Lateral thoracic radiograph from a dog. Notice the increased apicobasilar dimension causing dorsal displacement of carina, enlargement of left atrium and auricle, and dorsolateral displacement of caudal bronchi.
On the ventrodorsal and dorsoventral projections, an enlarged left atrium summates as an ovoid soft tissue opacity over the mid-caudal aspect of the cardiac silhouette. When enlarged, the left atrium can cause divergence or lateral displacement of the principle bronchi on this view. Sometimes left auricular (left atrial appendage) enlargement or displacement occurs concurrently in severe cases of left atrial enlargement. In these cases, the left auricle is seen as a soft tissue convexity on the cardiac silhouette at the 2-3 o’clock position on the ventrodorsal or dorsoventral projections.
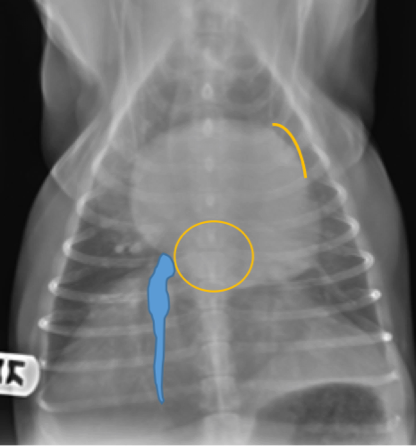
Figure 9. Ventrodorsal thoracic radiograph from a dog. Notice the enlarged left atrium summates as an ovoid soft tissue opacity over the mid-caudal aspect of the cardiac silhouette. Sometimes left auricular (left atrial appendage) enlargement or displacement occurs concurrently in severe cases of left atrial enlargement. In these cases, the left auricle is seen as a soft tissue convexity on the cardiac silhouette at the 2-3 o’clock position on the ventrodorsal or dorsoventral projection.
When evaluating a patient for cardiac disease, one must also pay attention to the size and shape of the vasculature. Pulmonary venous distension occurs without arterial enlargement with cardiac volume overload (e.g. left heart failure), myocardial failure (e.g. DCM, HCM) or left atrial obstruction secondary to a mass or thrombus. When a diagnosis of acute left sided congestive heart failure is made based on clinical signs, radiographs and possibly echocardiogram, treatment should be initiated immediately. Treatment includes supplemental oxygen, diuretic therapy, such as Furosemide as boluses or as a CRI, as well as a venodilator such as Nitroglycerine paste.
Non-cardiogenic pulmonary edema can occur secondary to airway obstruction, seizures, electrocution, vasculitis or acute lung injury/acute respiratory distress syndrome. Clinical signs are usually severe enough that patients are oxygen dependent. Radiographically, it presents as bilateral, dorsal or diffuse, interstitial or alveolar infiltrate. In these patients there is rarely evidence of cardiomegaly or pulmonary venous congestion.
It is important to take into account the history, clinical signs and other radiographic signs to determine if a patient has non-cardiogenic or cardiogenic pulmonary edema. Patients with non-cardiogenic pulmonary edema will be treated supportively with supplemental oxygen and may need mechanical ventilation. If there is equivocal cardiomegaly, consider performing an echocardiogram for evaluation of the left atrium. Additionally, a Lasix trial can be attempted, and a diagnosis may be made based on response to treatment.
Pulmonary thromboembolism (PTE) is a common disease in dogs but is rare in cats. PTE is associated with numerous predisposing conditions causing hypercoagulability, blood flow stasis, or endothelial injury. Predisposing diseases include hyperadrenocorticism, neoplasia, immune mediated hemolytic anemia, heartworm disease, and protein losing enteropathy or nephropathy. The most common signs are dyspnea, tachypnea, and depression. Other signs include coughing or hemoptysis or both, cyanosis, syncope, collapse, and sudden death.
Radiographic signs may include early oligemia, which is decreased vascularity to a region of lung. However, typically we identify the secondary resultant pulmonary edema and atelectasis, which results in a focal alveolar pattern. The cardiovascular consequences of PTE are dependent upon the degree of pulmonary vascular occlusion. Significant pulmonary vascular occlusion leads to pulmonary arterial hypertension and increased right ventricular (RV) afterload. Severe, acute changes in RV afterload result in RV dilatation and dysfunction. Radiographically, right ventricular enlargement can cause the cardiac apex to be displaced dorsally from the sternum on lateral projections. On the VD and DV images, the enlarged right ventricle appears more rounded and protrudes more into the right hemi-thorax, giving the cardiac silhouette a reverse D appearance.
It is important not confuse the normal rounding of the right atrium on the dorsoventral projection as cardiomegaly. This is another example why it is important to use more than one projection to determine if there is cardiomegaly and chamber enlargement. Pulmonary hypertension associated with pulmonary thromboembolism will cause enlargement of the pulmonary lobar artery (or arteries) and main pulmonary artery enlargement. Main pulmonary artery enlargement as well as pulmonary hypertension, can be confirmed via echocardiography. If a diagnosis of PTE is made based on a predisposing condition, clinical signs and radiographic findings, then treatment should be initiated. Therapy is mostly supportive. Successful treatment for acute PTE is typically followed by chronic anticoagulant or antiplatelet therapy.
Diffuse Pulmonary Patterns
Diffuse pulmonary disease may be in the form of a bronchial pattern, or interstitial or alveolar pattern. Diffuse interstitial or alveolar patters may be due to vasculitis, Acute lung injury (ALI), acute respiratory distress syndrome (ARDS), atypical pneumonia, or neoplasia such as lymphoma. Atypical pneumonia can be associated with other underlying airway disease such as tracheal collapse, or Kennel cough. The patient may present with coughing, respiratory distress and fever. A definitive diagnosis is made with an airway wash. In some cases, Mycoplasma spp. is an agent identified in patients with diffuse pulmonary infiltrates. Other common pathogens can be found concurrently. Treatment includes appropriate antibiotic therapy and supplemental oxygen, as well as a cough suppressant.
Kennel cough or Canine infectious respiratory disease complex (CIRDC) is a common finding in young dogs and dogs with a history of exposure to a shelter or boarding facility. These dogs typically present with coughing and retching and may or may not have a fever. Radiographically there is a diffuse bronchial pattern. There may also be concurrent aspiration pneumonia. The most common causative agents are B. bronchiseptica, S. equi subspecies zooepidemicus, and Mycoplasma spp. This is a self-limiting disease, but treatment can include cough suppressant and appropriate antibiotics such as Doxycycline. If the patient does not respond to treatment within seven days, then airway sampling should be performed for cytologic evaluation, culture and Mycoplasma testing.
We hope these tips help you to better understand the types of lung patterns that can occur in dogs and cats as well as the possible causes.
References
- Powell, L. L., Rozanski, E. A., Tidwell, A. S. and Rush, J. E. (1999): A Retrospective Analysis of Pulmonary Contusion Secondary to Motor Vehicular Accidents in 143 Dogs: 1994 – 1997 Journal of Veterinary Emergency and Critical Care, 9: 127–136. doi: 10.1111/j.1476-4431.1999.tb00074.
- Tyburski, James G. MD; Collinge, Julianne D. MD; Wilson, Robert F. MD; Eachempati, Soumitra R. MD: Pulmonary Contusions: Quantifying the Lesions on Chest X-Ray Films and the Factors Affecting Prognosis, Journal of Trauma-Injury Infection & Critical Care, May 1999 – Volume 46 – Issue 5 – pp 833-838.
- Thrall, D.E.: Textbook of Veterinary Diagnostic Radiology 6th Edition, Elsevier Saunders, St. Louis, Missouri, 2013.
- Thrall, D.E., Robertson, I.D.: Atlas of Normal Radiographic Anatomy and Anatomic Variants in the Dog and Cat, Elsevier Saunders, St. Louis, Missouri, 2011.
- Buchanan JW, Bücheler J: Vertebral scale system to measure heart size in radiographs. J Am Vet Med Assoc, 1995; 206:194–199.
- R. Lappin, J. Blondeau, D. Boothe, E.B. Breitschwerdt, L. Guardabassi, D.H. Lloyd, M.G. Papich, S.C. Rankin, J.E. Sykes, J. Turnidge and J.S. Weese: Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases, Journal of Veterinary Internal Medicine, 31, 2, (279-294), (2017).
- Robert Goggs, Livia Benigni, Virginia Luis Fuentes, and Daniel L. Chan: Pulmonary thromboembolism, Journal of Veterinary Emergency and Critical Care, Volume 19, Issue 1, First published: 27 February 2009.

